How Waterless Dyeing Works
Dyeing is a water-intensive process
To achieve a rich color, dye particles must penetrate a fabric completely. We use a liquid solution called a solvent to evenly carry dye throughout a fabric. One of the most common solvents used for dyeing, painting, and other chemical reactions is water. But after the reaction is complete, how do we separate the excess dye particles from the water?
20 Gallons


Using CO2 instead of H20
To reduce the amount of water needed to dye our products, we focus on the pressure and temperature of boiling—turning the solvent from a liquid to gas, while leaving the dye behind. Known as the boiling point, this allows us to harness the unique boiling points of carbon dioxide (specifically “supercritical” carbon dioxide, which is more liquid than gas at high pressure).
By using pressurized carbon dioxide instead of water as our solvent in the dyeing process, and releasing it as a gas, we lower our energy use considerably and save over 130,000 of gallons of water every year.
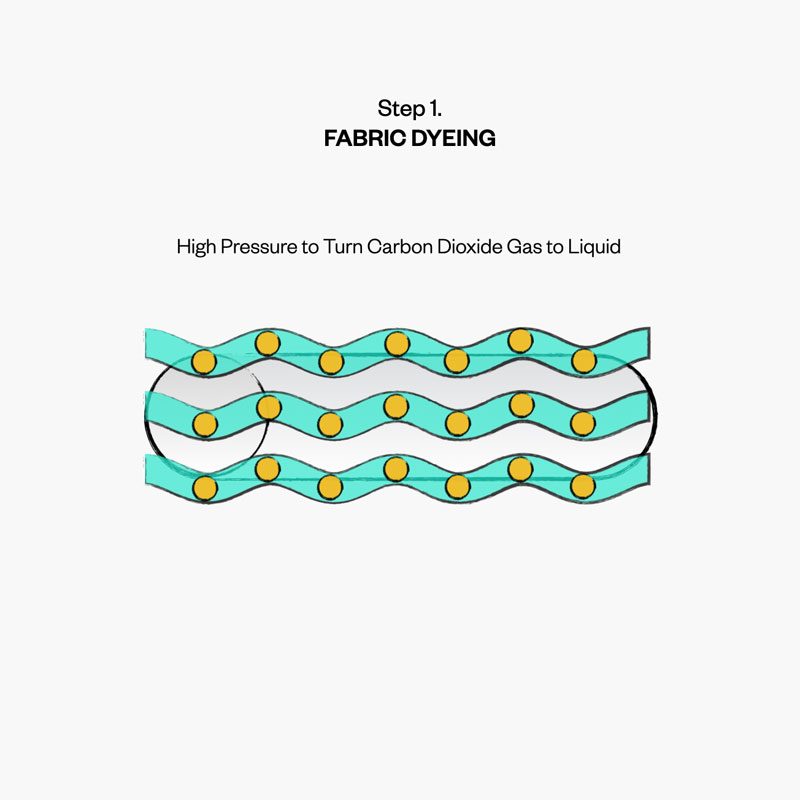
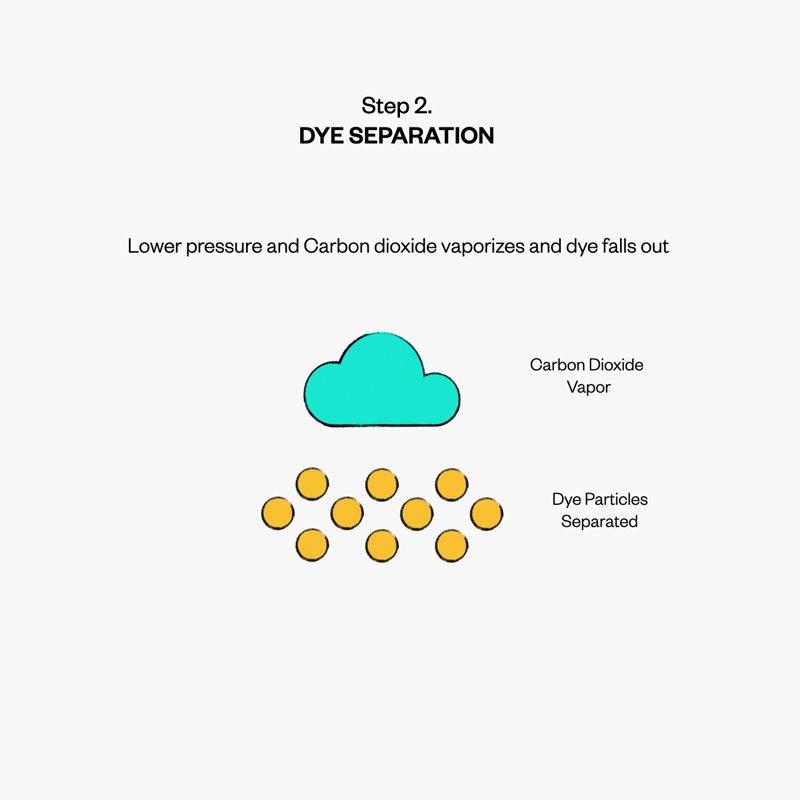
A good use for CO2: waterless dyeing
The carbon dioxide we use for waterless dyeing is captured from other processes in the fabric mill. When this carbon dioxide is separated in the dyeing process, instead of releasing it into the atmosphere, it can be re-captured and recycled nearly infinitely for future dye lots.
Carbon dioxide based waterless dyeing circumvents the need for large amount of water use, boiling or filtration—but it does require complex machinery. Fortunately our partners at Singtex (a leader in clean dyeing procedures for performance fabrics) have the necessary technology, and have been able to use this process in our Mercury, Doppler Merino and Responsive fabrics.
